TGA News: TGA targeting traditional medicine claims
The TGA is targeting medicines making traditional claims. Learn how to prepare your product range.
Read MoreZafe Zone Fined $39,960 for COVID-19 breaches
The TGA has fined Zafe Zone almost $40,000 for allegedly promoting its disinfectant as effective against coronavirus, without having the…
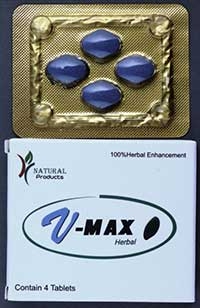
Read MoreSafety Advisory: V-Max Herbal Tablets
The TGA has issued an alert about V-MAX herbal tablets. These tablets were tested and found to contain the undeclared…
Read MoreTGA issues a $12,600 penalty for Caruso’s Natural Health product
Caruso’s Natural Health has been fined $12,600 for making false claims about holding evidence to support indications on their Caruso’s Kids Eye Care product.
Read MoreTGA News: TGA publishes listed medicine review outcome database
The TGA has published a database which lists the outcomes of reviews of listed medicines. This database makes information publicly…
Read MoreWe’re expanding! Servicing government departments from 2020
From 2020, our regulatory experts will start to offer services to local, state and territory, and federal government organisations. For…
Read MoreTGA News: You can now request consent not to comply with labelling order requirement
The TGA will provide consent not to comply with s 9(2) of TGO 92 where products meet eligibility criteria. This is an interim measure only.
Read MoreTGA News: TGA Extends Fee-Free Period for Permissible Indications Transition
The TGA has extended the fee-free period for sponsors of existing listed medicines to transition to using permitted indications until 6 March 2021
Read MoreTGA News: Consultation on Changes to the Permissible Ingredients Determination
The TGA is consulting on changes to the permissible ingredients determination. Submissions are due on 11 October 2019.
Read More- « Previous
- 1
- 2
- 3
- Next »